Definition
Is the adhesion of atoms, ions, or molecules from a gas, liquid, or dissolved solid to a surface.[1] This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid (the absorbate) permeates or is dissolved by a liquid or solid (the absorbent).[2] Note that adsorption is a surface-based process while absorption involves the whole volume of the material. The term sorption encompasses both processes, while desorption is the reverse of adsorption. It is a surface phenomenon.
Similar to surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent, or metallic) of the constituent atoms of the material are filled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature of the bonding depends on the details of the species involved, but the adsorption process is generally classified as physisorption (characteristic of weak van der Waals forces) or chemisorption (characteristic of covalent bonding). It may also occur due to electrostatic attraction.[3]
Adsorption is present in many natural physical, biological, and chemical systems, and is widely used in industrial applications such as activated charcoal, capturing and using waste heat to provide cold water for air conditioning and other process requirements (adsorption chillers), synthetic resins, increase storage capacity of carbide-derived carbons, and water purification. Adsorption, ion exchange, and chromatography are sorption processes in which certain adsorbates are selectively transferred from the fluid phase to the surface of insoluble, rigid particles suspended in a vessel or packed in a column. Lesser known, are the pharmaceutical industry applications as a means to prolong neurological exposure to specific drugs or parts thereof.
The word "adsorption" was coined in 1881 by German physicist Heinrich Kayser (1853-1940).[
Types of adsorption
Adsorption can be classified into two categories as described below,
(1) Depending upon the concentration : In adsorption the concentration of one substance is different at the surface of the other substance as compared to adjoining bulk or interior phase.
(i) Positive adsorption : If the concentration of adsorbate is more on the surface as compared to its concentration in the bulk phase then it is called positive adsorption.
Example :When a concentrated solution of KCl is shaken with blood charcoal, it shows positive adsorption.
(ii) Negative adsorption : If the concentration of the adsorbate is less than its concentration in the bulk then it is called negative adsorption.
Example :When a dilute solution of KCl is shaken with blood charcoal, it shows negative adsorption.
(2) Depending upon the nature of force existing between adsorbate molecule and adsorbent
(i) Physical adsorption : If the forces of attraction existing between adsorbate and adsorbent are Vander Waal’s forces, the adsorption is called physical adsorption. This type of adsorption is also known as physisorption or Vander Waal’s adsorption. It can be easily reversed by heating or decreasing the pressure.
Is the adhesion of atoms, ions, or molecules from a gas, liquid, or dissolved solid to a surface.[1] This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid (the absorbate) permeates or is dissolved by a liquid or solid (the absorbent).[2] Note that adsorption is a surface-based process while absorption involves the whole volume of the material. The term sorption encompasses both processes, while desorption is the reverse of adsorption. It is a surface phenomenon.
Similar to surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent, or metallic) of the constituent atoms of the material are filled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature of the bonding depends on the details of the species involved, but the adsorption process is generally classified as physisorption (characteristic of weak van der Waals forces) or chemisorption (characteristic of covalent bonding). It may also occur due to electrostatic attraction.[3]
Adsorption is present in many natural physical, biological, and chemical systems, and is widely used in industrial applications such as activated charcoal, capturing and using waste heat to provide cold water for air conditioning and other process requirements (adsorption chillers), synthetic resins, increase storage capacity of carbide-derived carbons, and water purification. Adsorption, ion exchange, and chromatography are sorption processes in which certain adsorbates are selectively transferred from the fluid phase to the surface of insoluble, rigid particles suspended in a vessel or packed in a column. Lesser known, are the pharmaceutical industry applications as a means to prolong neurological exposure to specific drugs or parts thereof.
The word "adsorption" was coined in 1881 by German physicist Heinrich Kayser (1853-1940).[
Types of adsorption
Adsorption can be classified into two categories as described below,
(1) Depending upon the concentration : In adsorption the concentration of one substance is different at the surface of the other substance as compared to adjoining bulk or interior phase.
(i) Positive adsorption : If the concentration of adsorbate is more on the surface as compared to its concentration in the bulk phase then it is called positive adsorption.
Example :When a concentrated solution of KCl is shaken with blood charcoal, it shows positive adsorption.
(ii) Negative adsorption : If the concentration of the adsorbate is less than its concentration in the bulk then it is called negative adsorption.
Example :When a dilute solution of KCl is shaken with blood charcoal, it shows negative adsorption.
(2) Depending upon the nature of force existing between adsorbate molecule and adsorbent
(i) Physical adsorption : If the forces of attraction existing between adsorbate and adsorbent are Vander Waal’s forces, the adsorption is called physical adsorption. This type of adsorption is also known as physisorption or Vander Waal’s adsorption. It can be easily reversed by heating or decreasing the pressure.
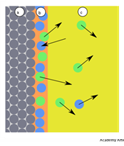
(ii) Chemical adsorption :If the forces of attraction existing between adsorbate particles and adsorbent are almost of the same strength as chemical bonds, the adsorption is called chemical adsorption. This type of adsorption is also called as chemisorption or Langmuir adsorption. This type of adsorption cannot be easily reversed.
Characteristics and general requirements
Activated carbon is used as an adsorbent
Adsorbents are used usually in the form of spherical pellets, rods, moldings, or monoliths with hydrodynamic diameters between 0.5 and 10 mm. They must have high abrasion resistance, high thermal stability and small pore diameters, which results in higher exposed surface area and hence high surface capacity for adsorption. The adsorbents must also have a distinct pore structure that enables fast transport of the gaseous vapors.
Most industrial adsorbents fall into one of three classes:
Factors which affect the extent of adsorption
The following are the factors which affect the adsorption,
(1) Nature of the adsorbate (gas) and adsorbent (solid)
(i) In general, easily liquefiable gases e.g., CO2, NH3, Cl2 and SO2 etc. are adsorbed to a greater extent than the elemental gases e.g. H2, O2, N2, He etc. (while chemisorption is specific in nature.)
(ii) Porous and finely powdered solid e.g. charcoal, fullers earth, adsorb more as compared to the hard non-porous materials. Due to this property powdered charcoal is used in gas masks.
(2) Surface area of the solid adsorbent
(i) The extent of adsorption depends directly upon the surface area of the adsorbent, i.e. larger the surface area of the adsorbent, greater is the extent of adsorption.
(ii) Surface area of a powdered solid adsorbent depends upon its particle size. Smaller the particle size, greater is its surface area.
(3) Effect of pressure on the adsorbate gas
(i) An increase in the pressure of the adsorbate gas increases the extent of adsorption.
Characteristics and general requirements
Activated carbon is used as an adsorbent
Adsorbents are used usually in the form of spherical pellets, rods, moldings, or monoliths with hydrodynamic diameters between 0.5 and 10 mm. They must have high abrasion resistance, high thermal stability and small pore diameters, which results in higher exposed surface area and hence high surface capacity for adsorption. The adsorbents must also have a distinct pore structure that enables fast transport of the gaseous vapors.
Most industrial adsorbents fall into one of three classes:
- Oxygen-containing compounds – Are typically hydrophilic and polar, including materials such as silica gel and zeolites.
- Carbon-based compounds – Are typically hydrophobic and non-polar, including materials such as activated carbon and graphite.
- Polymer-based compounds – Are polar or non-polar functional groups in a porous polymer matrix
Factors which affect the extent of adsorption
The following are the factors which affect the adsorption,
(1) Nature of the adsorbate (gas) and adsorbent (solid)
(i) In general, easily liquefiable gases e.g., CO2, NH3, Cl2 and SO2 etc. are adsorbed to a greater extent than the elemental gases e.g. H2, O2, N2, He etc. (while chemisorption is specific in nature.)
(ii) Porous and finely powdered solid e.g. charcoal, fullers earth, adsorb more as compared to the hard non-porous materials. Due to this property powdered charcoal is used in gas masks.
(2) Surface area of the solid adsorbent
(i) The extent of adsorption depends directly upon the surface area of the adsorbent, i.e. larger the surface area of the adsorbent, greater is the extent of adsorption.
(ii) Surface area of a powdered solid adsorbent depends upon its particle size. Smaller the particle size, greater is its surface area.
(3) Effect of pressure on the adsorbate gas
(i) An increase in the pressure of the adsorbate gas increases the extent of adsorption.
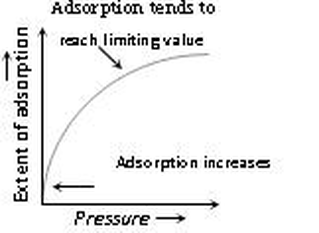
(ii) At low temperature, the extent of adsorption increases rapidly with pressure.
(iii) Small range of pressure, the extent of adsorption is found to be directly proportional to the pressure.
(iv) At high pressure (closer to the saturation vapour pressure of the gas), the adsorption tends to achieve a limiting value.
(iii) Small range of pressure, the extent of adsorption is found to be directly proportional to the pressure.
(iv) At high pressure (closer to the saturation vapour pressure of the gas), the adsorption tends to achieve a limiting value.
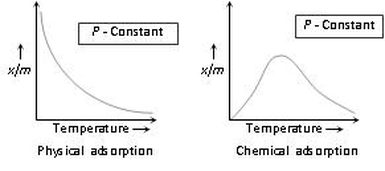
(4) Effect of temperature
(i) As adsorption is accompanied by evolution of heat, so according to the Le-Chatelier’s principle, the magnitude of adsorption should decrease with rise in temperature.
(ii)The relationship between the extent of adsorption and temperature at any constant pressure is called adsorption isobar.
(iii) A physical adsorption isobar shows a decrease in x/m (where ‘m’ is the mass of the adsorbent and ‘x’ that of adsorbate) as the temperature rises.
(iv) The isobar of chemisorption show an increase in the beginning and then decrease as the temperature rises.
ADSORPTION FROM SOLUTIONS
(1) The process of adsorption can take place from solutions also.
(2) In any solution, there are two (or more) components; solute and solvent. The solute may be present in the molecular or ionic form.
(3) The extent of adsorption from solution depends upon the concentration of the solute in the solution, and can be expressed by the Freundlich isotherm.
(4) The Freundlich adsorption isotherm for the adsorption from solution is, x/m = kc1/n where, x is the mass of the solute adsorbed, m is the mass of the solid adsorbent, c is the equilibrium concentration of the solute in the solution, n is a constant having value greater than one,
k is the proportionality constant, (The value of k depends upon the nature of solid, its particle size, temperature, and the nature of solute and solvent etc.)
(5) The plot of x/m against c is similar to that Freundlich adsorption isotherm. The above equations may be written in the following form, logx/m = 1lokg k + 1/n logc where c, is the equilibrium concentration of the solute in the solution.
APPLICATION OF ADSORPTION
The phenomenon of adsorption finds a number of applications. Important applications are given as follows.
(1) Production of high vacuum
(2) In Gas masks: This apparatus is used to adsorb poisonous gases (e.g. Cl2, CO oxide of sulphur etc.) and thus purify the air for breathing.
(3) For desiccation or dehumidification: These substances can be used to reduce/remove water vapours or moisture present in the air. Silica gel and alumina are used for dehumidification in electronic equipment.
(4) Removel of colouring matter from solution: (i) Animal charcoal removes colours of solutions by adsorbing coloured impurities. (ii) Animal charcoal is used as decolouriser in the manufacture of cane sugar.
(5) Heterogeneous catalysis: Mostly heterogeneous catalytic reactions proceed through the adsorption of gaseous reactants on solid catalyst. For example,
(i) Finely powdered nickel is used for the hydrogenation of oils.
(ii) Finely divided vanadium pentaoxide (V2O5) is used in the contact process for the manufacture of sulphuric acid.
(6) Separation of inert gases: Due to the difference in degree of adsorption of gases by charcoal, a mixture of inert gases can be separated by adsorption on coconut charcoal at different low temperatures.
(7) Softening of hard water
(i) The hard water is made to pass through a column packed with zeolite (sodium aluminium silicate)
(ii) Ca++, Mg++ ions which are responsible for hardness, get adsorbed on zeolite, exchanging sodium ions.
Na2Al2Si2O5 + CaCl2 —> CaAl2Si2O8 + 2NaCl
(iii) The exhausted zeolite is regenerated with 10% of sodium chloride solution.
CaAl2Si2O8 + 2NaCl —> Na2Al2Si2O8 + CaCl2
(8) De-ionisation of water
(i) Water can be de-ionised by removing all dissolved salts with the help of cation and anion-exchanger resin.
(ii) Cation-exchanger is an organic synthetic resin such as polystyrene-containing a macroanion (R—SO–3 etc)which has adsorbed H+ ions.
(iii) A resin containing a basic group (R3Na+ etc.) which has adsorbed OH– ions acts as anion exchanger.
(9) In curing diseases: A number of drugs are adsorbed on the germs and kill them or these are adsorbed on the tissues and heat them.
(10) Cleaning agents: Soap and detergents get adsorbed on the interface and thus reduce the surface tension between dirt and cloth, subsequently the dirt is removed from the cloth.
(11) Froth floatation process
A low grade sulphide ore is concentrated by separating it from silica and other earthy matter by this method.
(12) In adsorption indicators
Surface of certain precipitates such as silver halide, have the property of adsorbing some dyes like eosin, fluorescein etc.
(13) Chromatographic analysis
The phenomenon of adsorption has given an excellent technique of analysis known as chromatographic analysis.
(14) In dyeing: Many dyes get adsorbed on the cloth either directly or by the use of mordants.
Refrences
1- "Glossary". The Brownfields and Land Revitalization Technology Support Center. Retrieved 2009-12-21.
2- "absorption (chemistry)". Memidex (WordNet) Dictionary/Thesaurus. Retrieved 2010-11-02
3- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. (2010). "Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements". J Colloid Interface Sci. 347 (1): 15–24. doi:10.1016/j.jcis.2010.03.005. PMID 20356605.
WAS THIS INFORMATION USEFUL?
(i) As adsorption is accompanied by evolution of heat, so according to the Le-Chatelier’s principle, the magnitude of adsorption should decrease with rise in temperature.
(ii)The relationship between the extent of adsorption and temperature at any constant pressure is called adsorption isobar.
(iii) A physical adsorption isobar shows a decrease in x/m (where ‘m’ is the mass of the adsorbent and ‘x’ that of adsorbate) as the temperature rises.
(iv) The isobar of chemisorption show an increase in the beginning and then decrease as the temperature rises.
ADSORPTION FROM SOLUTIONS
(1) The process of adsorption can take place from solutions also.
(2) In any solution, there are two (or more) components; solute and solvent. The solute may be present in the molecular or ionic form.
(3) The extent of adsorption from solution depends upon the concentration of the solute in the solution, and can be expressed by the Freundlich isotherm.
(4) The Freundlich adsorption isotherm for the adsorption from solution is, x/m = kc1/n where, x is the mass of the solute adsorbed, m is the mass of the solid adsorbent, c is the equilibrium concentration of the solute in the solution, n is a constant having value greater than one,
k is the proportionality constant, (The value of k depends upon the nature of solid, its particle size, temperature, and the nature of solute and solvent etc.)
(5) The plot of x/m against c is similar to that Freundlich adsorption isotherm. The above equations may be written in the following form, logx/m = 1lokg k + 1/n logc where c, is the equilibrium concentration of the solute in the solution.
APPLICATION OF ADSORPTION
The phenomenon of adsorption finds a number of applications. Important applications are given as follows.
(1) Production of high vacuum
(2) In Gas masks: This apparatus is used to adsorb poisonous gases (e.g. Cl2, CO oxide of sulphur etc.) and thus purify the air for breathing.
(3) For desiccation or dehumidification: These substances can be used to reduce/remove water vapours or moisture present in the air. Silica gel and alumina are used for dehumidification in electronic equipment.
(4) Removel of colouring matter from solution: (i) Animal charcoal removes colours of solutions by adsorbing coloured impurities. (ii) Animal charcoal is used as decolouriser in the manufacture of cane sugar.
(5) Heterogeneous catalysis: Mostly heterogeneous catalytic reactions proceed through the adsorption of gaseous reactants on solid catalyst. For example,
(i) Finely powdered nickel is used for the hydrogenation of oils.
(ii) Finely divided vanadium pentaoxide (V2O5) is used in the contact process for the manufacture of sulphuric acid.
(6) Separation of inert gases: Due to the difference in degree of adsorption of gases by charcoal, a mixture of inert gases can be separated by adsorption on coconut charcoal at different low temperatures.
(7) Softening of hard water
(i) The hard water is made to pass through a column packed with zeolite (sodium aluminium silicate)
(ii) Ca++, Mg++ ions which are responsible for hardness, get adsorbed on zeolite, exchanging sodium ions.
Na2Al2Si2O5 + CaCl2 —> CaAl2Si2O8 + 2NaCl
(iii) The exhausted zeolite is regenerated with 10% of sodium chloride solution.
CaAl2Si2O8 + 2NaCl —> Na2Al2Si2O8 + CaCl2
(8) De-ionisation of water
(i) Water can be de-ionised by removing all dissolved salts with the help of cation and anion-exchanger resin.
(ii) Cation-exchanger is an organic synthetic resin such as polystyrene-containing a macroanion (R—SO–3 etc)which has adsorbed H+ ions.
(iii) A resin containing a basic group (R3Na+ etc.) which has adsorbed OH– ions acts as anion exchanger.
(9) In curing diseases: A number of drugs are adsorbed on the germs and kill them or these are adsorbed on the tissues and heat them.
(10) Cleaning agents: Soap and detergents get adsorbed on the interface and thus reduce the surface tension between dirt and cloth, subsequently the dirt is removed from the cloth.
(11) Froth floatation process
A low grade sulphide ore is concentrated by separating it from silica and other earthy matter by this method.
(12) In adsorption indicators
Surface of certain precipitates such as silver halide, have the property of adsorbing some dyes like eosin, fluorescein etc.
(13) Chromatographic analysis
The phenomenon of adsorption has given an excellent technique of analysis known as chromatographic analysis.
(14) In dyeing: Many dyes get adsorbed on the cloth either directly or by the use of mordants.
Refrences
1- "Glossary". The Brownfields and Land Revitalization Technology Support Center. Retrieved 2009-12-21.
2- "absorption (chemistry)". Memidex (WordNet) Dictionary/Thesaurus. Retrieved 2010-11-02
3- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. (2010). "Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements". J Colloid Interface Sci. 347 (1): 15–24. doi:10.1016/j.jcis.2010.03.005. PMID 20356605.
WAS THIS INFORMATION USEFUL?
 RSS Feed
RSS Feed